- Tel: 858.663.9055
 Email: info@nsjbio.com
Email: info@nsjbio.com
- Tel: 858.663.9055
- Email: info@nsjbio.com
The endoplasmic reticulum (ER) is a crucial organelle found in eukaryotic cells, playing a vital role in the synthesis, folding, modification, and transport of proteins and lipids. The ER is involved in numerous cellular processes, including calcium storage, lipid metabolism, protein folding, and the regulation of intracellular signaling. Disturbances in ER function are associated with a wide range of diseases, including neurodegenerative disorders, cancer, diabetes, and cardiovascular diseases.
Endoplasmic Reticulum Antibodies provide essential tools for investigating the structure, function, and stress responses of the ER in health and disease. These antibodies help researchers study how the ER interacts with other cellular components and how it contributes to the regulation of cell homeostasis.
Several key proteins are involved in the diverse functions of the endoplasmic reticulum. These proteins help maintain the structure and function of the ER, as well as mediate its response to cellular stress. Some important Endoplasmic Reticulum Antibodies target the following proteins:
Calnexin: A chaperone protein located in the ER that assists in the proper folding of newly synthesized proteins. Endoplasmic Reticulum Antibodies to calnexin help monitor protein folding and ER stress.
GRP78 (BiP): A heat shock protein that is a central player in the ER stress response. It helps to prevent misfolded proteins from accumulating within the ER, preventing cellular damage. Endoplasmic Reticulum Antibodies targeting GRP78 are useful for studying the unfolded protein response (UPR).
Protein Disulfide Isomerase (PDI): An enzyme that catalyzes the formation and rearrangement of disulfide bonds in nascent proteins within the ER. Antibodies against PDI are used to monitor protein maturation and folding.
Sar1: A small GTPase involved in the early stages of ER-to-Golgi transport. Endoplasmic Reticulum Antibodies targeting Sar1 are critical for studying vesicle trafficking and membrane dynamics.
XBP1: A key transcription factor activated by the UPR in response to ER stress. Endoplasmic Reticulum Antibodies targeting XBP1 help study ER stress and the cell's adaptive mechanisms.
These proteins and their associated functions make the endoplasmic reticulum integral to many aspects of cellular biology, from protein synthesis to stress responses and cellular homeostasis. Endoplasmic Reticulum Antibodies enable the precise investigation of these processes in research settings.
Endoplasmic Reticulum Antibodies are indispensable tools in cellular biology, helping researchers explore the structure, function, and stress responses of the ER. The following are common experimental techniques where Endoplasmic Reticulum Antibodies are applied to enhance understanding of ER-related processes:
Western blotting with Endoplasmic Reticulum Antibodies enables the detection of key proteins associated with the ER. By isolating proteins from cells or tissues, researchers can identify proteins involved in protein folding, ER stress, and the UPR, providing valuable insights into ER function and dysfunction.
Immunohistochemistry with Endoplasmic Reticulum Antibodies allows researchers to visualize the localization and distribution of ER-associated proteins in tissue sections. This technique is particularly useful for studying the expression patterns of ER proteins in different tissues and during disease states, such as cancer and neurodegenerative diseases.
Immunofluorescence with Endoplasmic Reticulum Antibodies enables the detection of ER proteins within living cells or fixed tissues. Fluorescent labeling of antibodies allows for high-resolution imaging of the ER network, highlighting changes in ER morphology, protein trafficking, and stress response pathways.
Flow cytometry with Endoplasmic Reticulum Antibodies allows researchers to analyze the expression levels of ER proteins at the single-cell level. This technique can be used to quantify ER protein abundance and examine how cells respond to ER stress or manipulate ER signaling pathways.
Chromatin immunoprecipitation (ChIP) with Endoplasmic Reticulum Antibodies can be used to study the interaction of ER proteins with chromatin. This method helps identify changes in gene expression associated with ER stress, UPR activation, and cellular adaptation to the stress response.
When researching the endoplasmic reticulum and its role in cellular function, using high-quality Endoplasmic Reticulum Antibodies is crucial. Our comprehensive selection of Endoplasmic Reticulum Antibodies is carefully validated for a variety of assays, offering researchers reliable results in the study of protein folding, ER stress, calcium regulation, and cellular homeostasis.
Whether you are investigating the molecular mechanisms of disease, studying ER function during development, or analyzing cellular responses to stress, Endoplasmic Reticulum Antibodies from NSJ Bioreagents provide the specificity, sensitivity, and versatility needed to advance your research.
Explore our extensive collection of Endoplasmic Reticulum Antibodies to accelerate your studies of the ER’s critical functions and its role in maintaining cellular health.
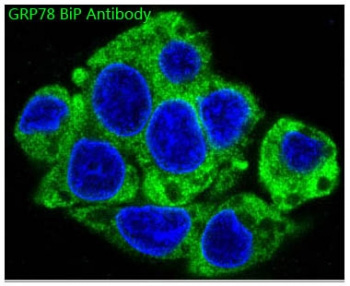
Immunofluorescent staining of FFPE human HepG2 cells with GPR78 / BiP antibody (green, cat # RQ5350) and DAPI nuclear stain (blue). HIER: steam section in pH6 citrate buffer for 20 min.
|
| ||

|